Down There (Part 3)
Bill Eberhard's Path from Spider Webs to Reinventing the Science of Sexual Selection
by Richard Conniff
The biologist who turned genitalia from an identification tool into a major field of science spent his career at the University of Costa Rica. At 62, when I met him in 2000, Bill Eberhard had bright brown eyes, an easy sense of humor, and the calm demeanor of a good family doctor. He did not much worry if other people thought his interests a little odd. One day on the road behind his house near San Jose, for instance, he stopped in mid-conversation at a mass of fly maggots wriggling their way across the pavement. He scooped them up with fingers and bits of leaves, put them in a baggie, and tucked them in his pants pocket for further study. Then he leapt into the air to catch one of the adult flies.
Eberhard’s lifelong ambition has been to walk in the woods and know what he is looking at, and he has made a career of discovering amazing behaviors other people move too fast to notice. He once identified a new species of spider, for instance, that throws a spitball on a thread to catch and reel in moths for dinner. He named the species dizzydeani, after the baseball pitcher.
Eberhard, who grew up in Arizona, started paying attention to genitalia as a graduate student at Harvard in the 1960s. The taxonomists he hung around with often relied on telltale differences in genitalia to identify species within the narrow animal groups they studied. But “nobody realized,” says Eberhard, that extreme variation in the genitalia is “one of the most basic tendencies in all of animal evolution,” occurring in species “from nematodes to mammals.”
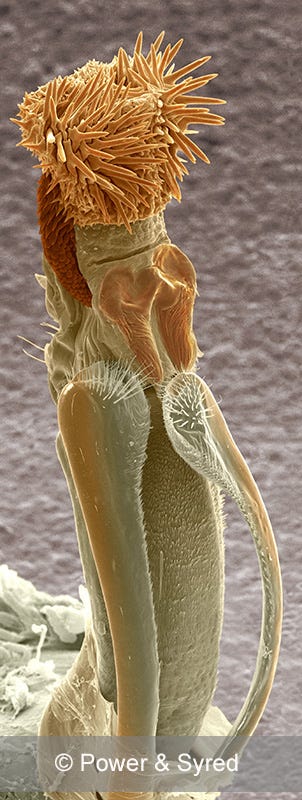
Spiders, Eberhard’s specialty, happened to make an excellent introduction to the study of genitalia. Because spiders typically hang upside down in webs, sex is easier to observe. (“Use of a mirror,” Eberhard writes, “also allows detailed observations of species without webs.”) Male spiders also have the curious habit of masturbating, in effect, and loading the sperm into a tubular arm-like appendage called the pedipalp, which they insert into the female. The female genitalia, often ignored in other animal groups, have a lot of external parts and tend to be well described in the scientific literature. So in the course of his studies, Eberhard naturally took note of the groping, hammering, twisting, vibrating, and rhythmic expansion of parts on both sides, and of course “repeated insertions and withdrawals” that constitute spider bliss.
He went on to study genitalia for its own sake in the early 1980s. Eberhard and his wife Mary Jane West-Eberhard, herself a prominent biologist, spent a semester as visiting fellows at the University of Michigan, and he took advantage of the library there to read about genitalia-- first fish week, then reptile week, then mammals, and so on. The result was his 1985 book, Sexual Selection and Animal Genitalia. It launched a flood of research on the topic, with dozens of new papers now published annually and ferocious debate over what it all means.
When past biologists tried to explain the bizarre variety of genitalia in the natural world, they sometimes argued that it was all a strange accident. Those claspers, feather dusters, and french ticklers couldn’t possibly be functional, could they? They were just a byproduct of changes elsewhere in the body.
Other researchers conceded that they might be useful, but for “lock-and-key” purposes. Complicated genitalia supposedly served the female as a built-in chastity belt. Only males with the right key, in the form of correspondingly complicated genitalia, could get inside to fertilize her eggs. Eberhard pointed out the flaws in this explanation: Female genitalia are often soft and not suited to mechanically blocking males of closely-related species. Lock-and-key theory also implied incredible stupidity. The female supposedly had to wait until Mr. Right was trying to put his key in her lock before it dawned on her that he was actually Mr. Oh So Wrong.
By the 1980s, biologists knew better. Feminism and the sexual revolution had shaken up old male attitudes. Scientists had also rediscovered the importance of female choice in shaping evolution. Charles Darwin himself had proposed that species evolve not just by natural selection (the ability of some individuals to stay alive when others are getting killed), but also by sexual selection (the ability of some individuals to attract a mate when others are getting shot down in flames). Darwin thought sexual selection would be a relatively mild force for evolutionary change, because getting rejected isn’t as bad as getting killed, is it?
But modern biologists think maybe it is that bad, after all. The competition to attract a mate and produce offspring is brutal, and it can cause rapid changes in the shape and behavior of a species. Females typically have the final say about sex, and they tend to choose the bird with the brightest feathers, or the frog with the loudest croak.
And the male with the weirdest genitalia?
Eberhard argued that the extreme variation in animal genitalia didn’t happen by accident or to keep things under lock-and-key. It was sexual selection. After the flashy external displays have done their part, he said, the competition to impress or manipulate the female continues internally, during the act of copulation and beyond. Male genitalia tend to evolve faster than any other trait in the animal world because a new gizmo there can directly improve a male’s chances of inseminating the female’s eggs. And that means the gene for the gizmo proliferates in the next generation.
The evidence that sexual selection favors new, improved genitalia is now overwhelming: For one thing, variations occur mainly where they are likely to be useful—that is, in species where male and female actually get together for internal fertilization. (It’s much less common in species where eggs and sperm mingle unceremoniously on the ocean currents. For them, fancy genitalia would be about as useful as a new bicycle.) Male genitalia evolve twice as fast in species where the female mates with more than one male. In those cases, the need to one-up rivals apparently gives an advantage to the male who can offer a female that certain something extra. But biologists disagree sharply about what that something extra is actually doing for, or to, the female.
On one side of the debate, Eberhard emphasizes cooperation. The challenge for the female, he says, is to choose what she regards as the best male to father her offspring, and male genitalia evolve as “internal courtship devices” to help sway the decision. A male with the right moves, or a knack for scratching a certain itch, is less likely to get kicked out of bed before he has fertilized the female’s eggs. He may be able to induce certain muscle contractions which encourage sperm transport. Or he may leave the female more willing to lay eggs fertilized by his sperm and less interested in racing off to mate with some other male.
When Eberhard describes couples mating, he emphasizes the nuances of sexual gratification, and he brings his acute naturalist’s eye to the task. Where ordinary folk might say, “Ugh a spider,” and turn away, for instance, he sees a male and female of a South American species flexing and sighing in contrapuntal rhythm. “The strongest muscles are in the pedipalps and he inserts both of them into the female, one of them braced against the other, and starts squeezing rhythmically.” The female apparently likes this type of internal courtship. But at certain points, “she sings. She makes a squeaky leather kind of sound, usually just as the male squeezes her tightest, and he responds by relaxing.” It’s a kind of copulatory dialogue, Eberhard says. Males that are more attentive to the female, more cooperative in terms of relaxing, tend to father more of her offspring.
(To be continued tomorrow: Copulatory Dialogues? Or Warfare by Other Means?)