To Fight Malaria, Disinfect Mosquitoes
They're just the messengers for the parasites that make us sick.
by Richard Conniff
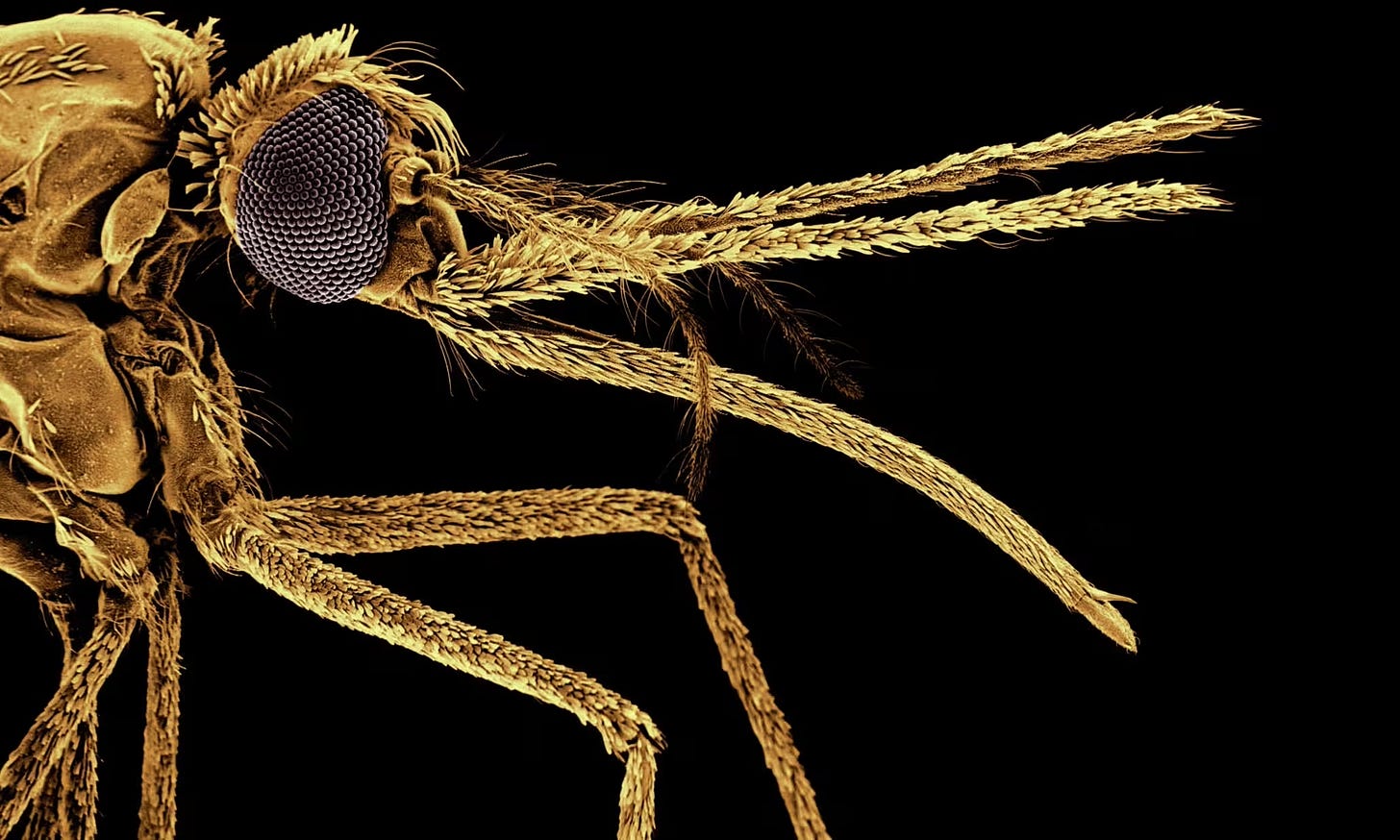
It was one of the great public health success stories of this century, until suddenly it wasn’t. The campaign, led by the World Health Organization, drove down malaria deaths by almost half, from an estimated 839,000 people worldwide in 2000 to just 438,000 in 2015. Then progress stalled, and malaria began to creep back and assert its old power.
This week in the journal Nature, researchers at Harvard’s School of Public Health deliver a novel approach that promises to save lives and put progress against malaria back on track. I’ll get to the details in a moment, but first some background.
Malaria is humanity’s most ancient and widespread public health enemy. By one estimate, it has killed half of all humans who ever lived. The Anopheles mosquitoes that transmit the disease occur on every continent except Antarctica, and climate change is steadily extending their range. In the United States in the nineteenth century, before effective mosquito control, the disease was killing people as far north as the Great Lakes. Over the past two years, for the first time in decades, locally-acquired cases have again occurred here, in Florida, Texas, Arkansas, and Maryland.
When you see that word “people” now, though, think mainly about children under age five, mostly in sub-Saharan Africa. Victims of the disease become listless, with wasted limbs and swollen bellies from the parasites infesting the liver, and it is as if a cloud has descended over their minds. Children account for three-quarters of the dead in that region, and small coffins are in strong demand.

The WHO global anti-malaria campaign set out by attacking the traditional targets of malaria control: The Anopheles mosquitoes that deliver the malaria parasite to the bloodstream with their bite, and the Plasmodium parasite itself in the bodies of human victims. That meant bringing vulnerable areas rapid diagnostic tests, better drug treatments, and, above all, improved and more abundant insecticide-treated nets to kill the Anopheles mosquitoes.
The reason the campaign went flat was also traditional: Natural selection favors mosquitoes resistant to insecticides, and it encourages the proliferation of malaria parasites resistant to new treatments in the human body. It’s happened like this before, notably in the 1950s when the insecticide DDT produced a stunning reduction in malaria cases, followed in the 1960s by a devastating resurgence of the disease.
The new study from Harvard researchers delivers an entirely untraditional approach: Instead of using bed nets just to kill mosquitoes, the researchers propose adding a chemical that kills the malaria parasite, too, within the mosquito’s body. “And then even if that mosquito lives,” says Alexandra S. Probst, “it’s still is not going to go on to transmit malaria. So that was really the rationale for this approach.”
Probst, who just earned her PhD this month, shares first author credit on the study with Douglas G. Paton, a former post-doc at Harvard, now at the University of Georgia. Both worked under malaria specialist Flaminia Catteruccia, who first dreamed up the new method as a way to break through the current stalemate in the fight against malaria. She and Paton proposed a first draft of the idea in 2019.
Proving that it can work meant starting with 81 compounds previously shown to be effective against the parasite in past human or animal studies and then subjecting them to a series of tests: Would they work in a bed net the way an insecticide works, from a brief contact with a mosquito’s legs? Would they still kill the parasite in combination with insecticides? Would they remain effective through a net manufacturing process that involves heating the material past 300 degrees Fahrenheit?
It wasn’t possible to answer such questions the easy way, by testing the parasite outside its host in a petri dish. “So this screen was done entirely in vivo in the mosquito, and that was, yeah, a bit of a challenge to optimize,” says Probst. “This basically involved, you know, directly pipetting our drug of interest onto mosquitoes one at a time.” Over and over and over. Demonstrating the effectiveness of a drug also required the researchers to dissect each mosquito and count how many Plasmodium oocysts, the early stage of the parasite’s development, remained in its gut after treatment.
Of the original 81 compounds, just two made the final cut. Both are variants on a drug first developed in the 1940s, which showed anti-malarial potential in the laboratory back then, but failed to work in living animals. Both variants came from Oregon Health & Science University, where researchers used modern laboratory methods to understand how the original drug did or didn’t work in different circumstances. Then they made chemical modifications to improve on the original. In the new study, the more effective of the two compounds killed 100 percent of malaria parasites present in the bodies of mosquitoes within just six minutes after contact.
This method is less likely to lead to resistance, according to Probst and her co-authors, in part because it appears to have no effect on the behavior of mosquitoes. Combining the anti-Plasmodium treatment with insecticides on the net will still kill as many of the mosquitoes as possible. But the survivors will simply be disinfected—cured!—and no longer able to transmit malaria with their bites.
Resistance is also less likely because natural selection is a numbers game. When an insecticide encounters billions of mosquitoes, a few of the mosquitoes may survive because of ordinary mutations. These resistant mosquitoes gradually come to dominate the population as the insecticide kills off their more vulnerable counterparts. The same numbers game happens with an anti-malaria drug encountering millions of Plasmodium cells in a human body. But in the gut of the mosquito, there may be just a few dozen to a few hundred Plasmodium oocysts.
Both anti-malarial compounds appear to be easily scalable, cheap to synthesize, and long-lasting. Harvard is currently negotiating with net manufacturers for further testing and potentially for broader deployment. “I don't think this is the end-all, be-all tool to eradicate malaria,” says Probst. But it will be “a complementary tool in an arsenal that includes drugs for people, drugs for mosquitoes, better nets, better draining of larval breeding sites, and, of course, vaccines, which have now recently been approved for malaria in the last couple years. We're really going to need a combination of many approaches.”
Putting the best combination to work against malaria has never been more urgent. In January, Donald Trump’s billionaire consigliere Elon Musk disbanded USAID (the U.S. Agency for International Development) and abruptly ended U.S. funding for anti-malaria programs abroad. That’s put an $800-million hole in the budget, and will likely push malaria deaths this year well past the 600,000 mark.
According to a website created by university researchers, those cuts will cause 78,800 additional malaria deaths in children over the first year. That’s one every seven minutes. Speaking of Musk, Bill Gates, who has devoted his fortune largely to the fight for public health, recently commented, “The picture of the world’s richest man killing the world’s poorest children is not a pretty one.”
Combine that with a president who is more interested in waging war against science, and particularly against Harvard, rather than against human disease, and the fight to stop malaria is certain to get much harder.
Richard Conniff’s latest book is Ending Epidemics: A History of Escape from Contagion.






Gates's comment says says it all. Thanks for this essay, Dick.
Musk and Trump could be charged and convicted of conspiracy to commit murder. The World Court should charge them along with the auttours of Project 2025.